![]()
Collaborative Research Center/Transregio 63
"Integrated Chemical Processes in Liquid Multiphase Systems"
"Integrated Chemical Processes in Liquid Multiphase Systems"
![]()
Collaborative Research Center/Transregio 63
"Integrated Chemical Processes in Liquid Multiphase Systems"
Sub-Coordinators: Prof. Dr.-Ing. Andreas Seidel-Morgenstern Prof. Dr.-Ing. Christof Hamel
Researcher: M. Sc. Sabine Kirschtowski
During the first founding period we identified the reaction network for the hydroformylation of the long-chain olefin 1-decene, catalytic cycles with all main and side reaction were developed and mechanistic kinetic models were derived. These were reduced by subset-selection techniques and validated successfully. The second founding period was characterized by the study of solvent composition effects and the effects of variable chain length of the substrates. Thereby 1-decene, a model substrate for petro and oleo chemicals, was used to investigate the sub network of the isomerisation in detail separately and the tandem reaction consisting of isomerisation and hydroformylation (so called isomerizing hydroformylation). The results were applied for an extension of the catalytic cycle and revision of the mechanistic models, which could be validated in a broad range of temperatures and pressures, successfully. After adjusting the parameters it was also possible to apply the kinetic model to the hydroesterfication reaction.
In the third founding period the subproject A3 deals with the experimental and kinetic research concerning the mechanism and the kinetic of the hydroaminomethylation (HAM) as tandem reaction consisting of reductive amination (RA) and hydroformylation (Hyfo). Thus, the gained knowledge and developed methods for the hydroformylation should be used to analyse and quantify the kinetics.
To require detailed knowledge regarding the overall reaction the sub-network of the reductive amination step is analysed separately in a thermomorphic solvent system. The reaction network and the catalytic cycle will be clarified for the RA to formulate and define new kinetic models. Therefore, perturbation experiments, IR spectroscopy, design of experiments and parameter reduction techniques will provide a data basis. Based on an analysis of the sub network of RA, the next step will be the direct coupling of the olefin–hydroformylation and the RA in a tandem reaction in order to formulate mechanistic kinetic models (Figure 1). Additionally, transient studies will be performed by imposed time-varying concentrations, temperatures and catalyst-ligand properties for olefins with internal double bonds. The developed kinetic models for the tandem reaction of the hydroaminomethylation should provide a basis for the design of reactors and processes. Quantitative assessment of catalyst deactivation processes through feed contamination or addition of water as well as the development of general rules for evaluation of tandem reactions (multi-pot vs. single-pot synthesis) will complete this work package.
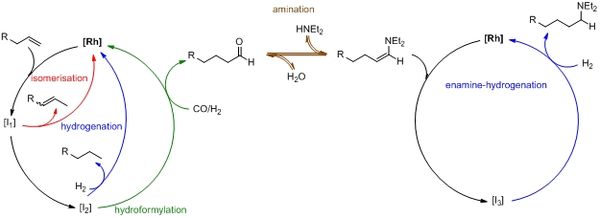
Figure 1: Hydroamiomethylation as tandem reaction from reductive amination and hydroformylation
Hamel, C.; Seidel-Morgenstern, A. Potential of membranes for process intensification of selective oxidations on catalyst, reactor and total process level. Chemie Ingenieur Technik, 94, 1-2, 1-15, 2022. [DOI: 10.1002/cite.202100130]
Kortuz, W.; Kirschtowski, S.; Seidel-Morgenstern, A.; Hamel, C. Kinetics of the Rhodium-Catalyzed Hydroaminomethylation of 1-Decene in a Thermomorphic Solvent System. Chemie Ingenieur Technik, (in press), 2022. [DOI:10.1002/cite.202100180]
Mueller, I.; Runne, E.; Hamel, C. Comparative Study on Mechanistic Kinetic Modeling of the Enzymatic Synthesis of Galacto-Oligosaccharides. Chemie Ingenieur Technik, (in press), 2022. [DOI:10.1002/cite.202100190]
Brune, A.; Geschke, A.; Seidel-Morgenstern, A.; Hamel, C. Modelling and Simulation of Catalyst Deactivation during Propane Dehydrogenation - Comparison of Different Modelling Approaches. Chemical Engineering and Processing: Process Intensification, 108689, 2021. [DOI:10.1016/j.cep.2021.108689]
Kirschtowski, S.; Jameel, F.; Stein, M.; Seidel-Morgenstern A.; Hamel, C. Kinetics of the Reductive Amination of 1-Undecanal in Thermomorphic Multicomponent System, Chemical Engineering Science, 230, 116187, 2021. [doi:10.1016/j.ces.2020.116187]
Walter, J.P.; Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Stationary and Transient Modelling of a Heat-Integrated Membrane Reactor for the Propane Dehydrogenation. Catalysts, 11, 1056, 2021. [DOI:10.3390/catal11091056]
Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Analysis and Model-Based Description of Deactivation and Regeneration of a VOx Catalyst for Selective Dehydrogenation of Propane, Catalysts – Special Issue: Design of Heterogeneous Catalysts and Adsorbens, 10, 1374, 2020. [doi:10.3390/catal10121374]
Felischak, M.; Wolff, T.; Alvarado Perea, L.; Seidel-Morgenstern, A.; Hamel, C. Detailed Kinetic Model for the Reaction of Ethene to Propene on Ni/AlMCM-41. Chem. Ing. Tech., 92(5), 564-574, 2020. [doi.org/10.1002/cite.201900139]
Kirschtowski, S.; Kadar, C.; Seidel-Morgenstern, A.; Hamel C. Kinetic Modeling of Rhodium-Catalyzed Reductive Amination of Undecanal in Different Solvent Systems. Chem. Ing. Tech., 92(5), 582-588, 2020. [doi.org/10.1002/cite.201900135]
Brune, A.; Wolff, T.; Seidel‐Morgenstern, A.; Hamel, C. Analysis of Membrane Reactors for Integrated Coupling of Oxidative and Thermal Dehydrogenation of Propane; Chem. Ing. Tech., 91(9), 645, 2019. [DOI: 10.1002/cite.201800184]
Felischak, M.; Wolff, T.; Alvarado Perea, L.; Seidel-Morgenstern, A.; Hamel, C. : Influence of process parameters on single bed Ni/(Al)MCM-41 for the ethene to propene reaction. Chem. Eng. Sci., 201, 2019. [doi.org/10.1016/j.ces.2019.115246]
Gerlach, M.; Kirschtowski, S.; Seidel-Morgenstern, A.; Hamel, C. Kinetic Modeling of the Palladium-Catalyzed Isomerizing Methoxycarbonylation of 1-Decene. Chem. Ing. Tech., 90(5), 673-678, 2018. [doi.org/10.1002/cite.201700162]
Gaide, T.; Jörke, A.; Schlipköter, K.E.; Hamel, C.; Seidel-Morgenstern, A.; Behr, A.; Vorholt, A. J. Isomerization/hydroformylation tandem reaction of a decene isomeric mixture with subsequent catalyst recycling in thermomorphic solvent systems. Appl. Catal., A, 532, 50-56, 2017. [doi: http://doi.org/10.1016/j.apcata.2016.12.011]
Gerlach, M.; Wajid, D. A.; Hilfert, L.; Edelmann, F. T.; Seidel-Morgenstern, A.; Hamel, C. Impact of minor amounts of hydroperoxides on rhodium-catalyzed hydroformylation of long-chain olefins. Catal. Sci. Technol., 7, 1465-1469, 2017. [doi: 10.1039/C7CY00244K]
Janiga, G.; Stucht, D.; Bordás, R.; Temmel, E.; Seidel-Morgenstern, A.; Thévenin, D.; Speck, O. Non-invasive 4D flow characterization in a stirred tank via phase-contrast magnetic resonance imaging. Chem. Eng. Tech., 40, 1370–1327, 2017. [doi: 10.1002/ceat.201700067]
Jörke, A.; Gaide, T.; Behr, A.;Vorholt, A. J.; Seidel-Morgenstern, A.; Hamel, C. Hydroformylation and tandem isomerization–hydroformylation of n-decenes using a rhodium-BiPhePhos catalyst: Kinetic modeling, reaction network analysis and optimal reaction control. Chem. Eng.J., 313, 382-397, 2017. [doi: http://doi.org/10.1016/j.cej.2016.12.070]
Jörke, A.; Seidel-Morgenstern, A.; Hamel, C. Rhodium-BiPhePhos catalyzed hydroformylation studied by operando FTIR spectroscopy: Catalyst activation and rate determining step. J. Mol. Catal. A: Chem., 426, 10-14, 2017. [doi: http://dx.doi.org/10.1016/j.molcata.2016.10.028]
Lemberg, M.; Sadowski, G.; Gerlach, M.; Kohls, E.; Stein, M.; Hamel, C.; Seidel-Morgenstern, A. Predicting solvent effects on the 1-dodecene hydroformylation reaction equilibrium. AIChE J., Advance Article, 2017. [doi: 10.1002/aic.15782]
Gao, K.; Yang, J.; Seidel-Morgenstern, A.; Hamel, C. Methane Dehydro-Aromatization: Potential of a Mo/MCM-22 Catalyst and Hydrogene-Selective Membranes. Chem. Ing. Tech., 88(1-2), 168–176, 2016. [doi:10.1002/cite.201500139]
Jörke, A.; Kohls, E.; Triemer, S.; Seidel-Morgenstern, A.; Hamel, C.; Stein, M. Resolution of Structural Isomers of Complex Reaction Mixtures in Homogeneous Catalysis. Chem. Eng. Process., 102, 229-237, 2016. [doi:10.1016/j.cep.2016.01.001]
Kiedorf, G.; Wolff, T.; Seidel-Morgenstern, A.; Hamel, C. Kinetic Analysis oft he Hydrocarbon Total Oxidation Using Individually Measured Adsorption Isotherms. Chem. Ing. Tech., 88(11), 1746-1760, 2016. [doi: 10.1002/cite.201600043]
Chauhan, G.; Stein, M.; Seidel-Morgenstern, A.; Pant, K. K.; Nigam, K. D. P. The thermodynamics and biodegradability of chelating agents upon metal extraction. Chem. Eng. Sci., 137, 768-785, 2015. [doi:10.1016/j.ces.2015.07.02]
Hentschel, B.; Kiedorf, G.; Gerlach, M.; Hamel, C.; Seidel-Morgenstern, A.; Freund, H.; Sundmacher, K. Model-Based Identification and Experimental Validation of the Optimal Reaction Route for the Hydroformylation of 1-Dodecene. Ind. Eng. Chem. Res., 54(6), 1755-1765, 2015. [doi:10.1021/ie504388t]
Jörke, A.; Seidel-Morgenstern, A.; Hamel, C. Isomerization of 1-decene: Estimation of Thermodynamic Properties, Equilibrium Composition Calculation and Experimental Validation Using a Rh-Biphephos Catalyst. Chem. Eng. J., 260, 513-523, 2015. [doi:10.1016/j.cej.2014.09.015]
Jörke, A.; Triemer, S.; Seidel-Morgenstern, A.; Hamel, C. Kinetic Investigation Exploiting Local Parameter Subset Selection: Isomerization of 1-Decene using a Rh-Biphephos Catalyst. Chem. Ing. Tech., 87(6), 1-14, 2015. [doi:10.1002/cite.201400148]
Kiedorf, G. Mechanistic and kinetic analysis of homogeneously and heterogeneously catalyzed reactions. Otto-von-Guericke-Universität Magdeburg, 2018.
Jörke, A. Mechanisms and kinetics of petro- and oleochemicals in complex hydroformylation reaction networks. Otto-von-Guericke-Universität Magdeburg, 2018.
Temmel, E. Design of continuous crystallization processes. Otto-von-Guericke-Universität Magdeburg, 2016.
Hoang Minh, D. A simultaneous optimization approach to optimal experimental design.Technische Universität Berlin, 2014.